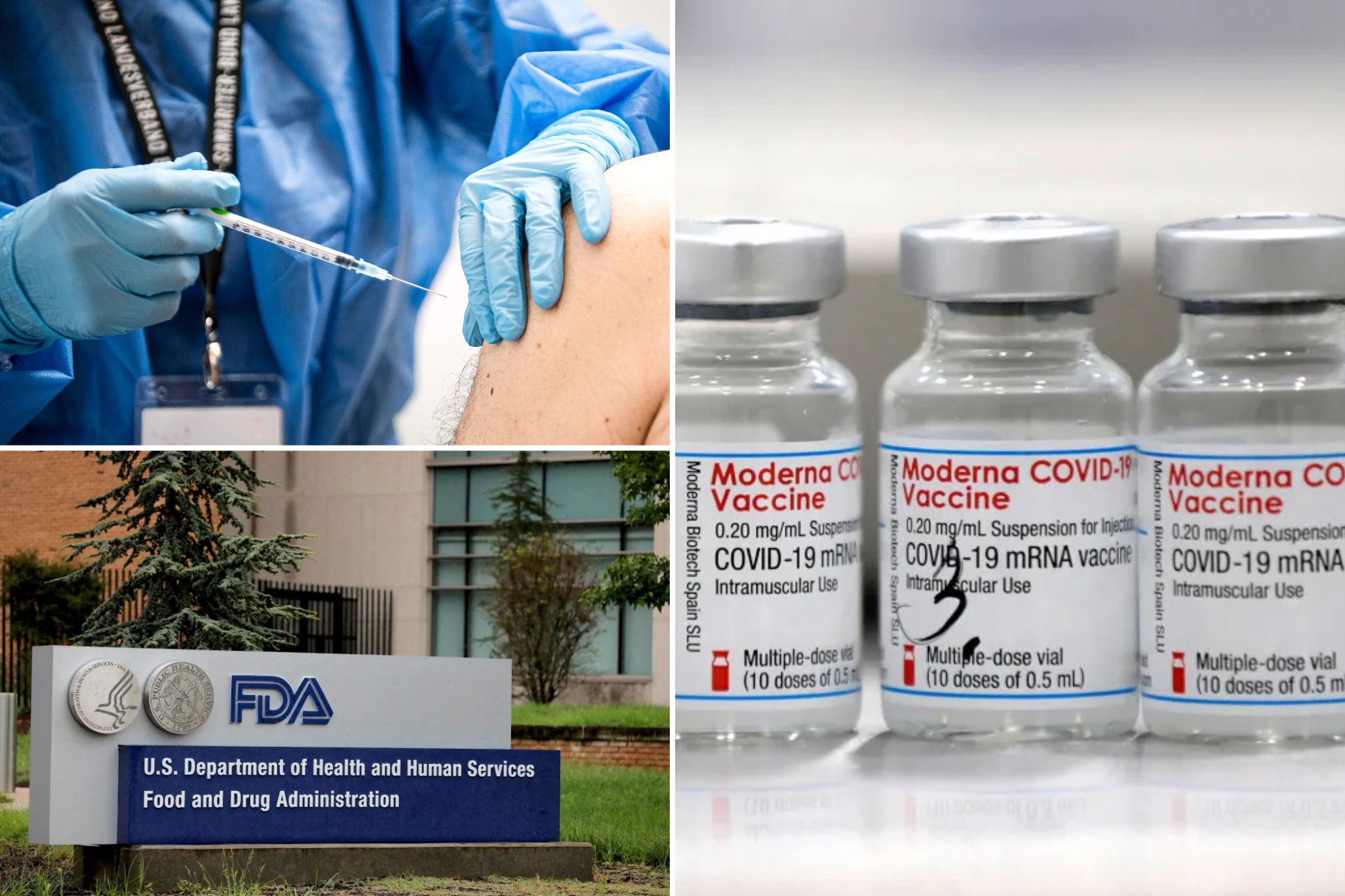
The United States Food and Drug Administration has approved the COVID-19 vaccine of the new generation of modernity for all 65 years old, and more, the company said on Saturday, the first guarantee since the regulator strengthened the requirements.
The vaccine has also been approved for people from 12 to 64 years old with at least one or more underlying risk factors defined by disease control and prevention centers, Moderna said in a statement.
The company said he hopes to have the vaccine, called Mnexspike, available for the 2025-2026 respiratory virus season.
“The FDA’s approval of the third product, MnexSpike, adds a new important tool to help protect people at a high risk of severe Covid-19 diseases,” said CEO Stephane Banel in the statement.
The Department of Health and Human Services, under the leadership of the skeptic of the vaccine for a long time, Robert F. Kennedy Jr., is hardening the regulatory scrutiny on vaccines.
The FDA said that on May 20, it was planned to require drug makers to test their covits reinforcement shots against an inert placebo in healthy adults under the age of 65 for approval, limiting them efficiently to adults and those who are at risk of developing serious illnesses.
The modern vaccine can be stored in refrigerators instead of freezers, to provide a longer useful life and facilitate distribution, especially in developing countries where supply chain problems could make it difficult for vaccination pipes.
The disease control and prevention centers, which Kennedy also monitored, said on Thursday that covid vaccines are still an option for healthy children when parents and doctors agree that it is necessary, stopping from the Kennedy announcement days before the agency would remove the traits of their immunization calendar.
The CDC’s announcement facilitates investors’ concerns to some extent, according to analysts, as it maintains the existing framework for adults and at risk people who generally look for the shots.
FDA leaders have said that 100 to 200 million Americans could still be eligible for annual traits.
Modern is committed to its most recent messaging RNA vaccines, as it claims to demand its original demand for the Covid Spikevax vaccine and a lower catchment than its expected of its vaccine against the respiratory syncitial virus.
Approval for MnexSpike was based on late -phase test data, which showed that the trait was not lower than effective compared to Spikevax in individuals 12 years and older.
The shot also showed a higher efficiency compared to Spikevax in adults of 18 years and more in the study.
Kennedy has begun a significant review of the Health Departments, and fired thousands of employees to align -with the aim of President Donald Trump to drastically reduce the federal government.
This has further ignited concerns about possible interruptions in the regulatory review of treatments and vaccines.
The CDC’s foreign group of vaccine experts in April discussed recommending reinforcement features only for populations at risk of Covid-19 serious for the next immunization campaign.
The FDA approved this month the Covid NuVaxovid vaccine of Novavax, limiting its use to adults over and older than 12 years with conditions that put them at risk due to the disease.
The conditions that constitute the additional risk from diseases such as diabetes and heart disease to behaviors such as physical inactivity and substance abuse, according to the CDC.
While modern traits, as well as Pfizer-Biontech Comirnaty, are based on the mRNA, the Novavax vaccine is based on protein and it takes more to be manufactured.
This month, Moderna withdrew an application that sought to approval of its combined flu and caveid vaccine to wait for effective data from a late stage test of its flu shot.
#FDA #approves #COVID #vaccine #generation #modern #adults #years #older
Image Source : nypost.com